Revolutionizing CNS Tumor Detection
CNSide™: Precision Diagnostics for Leptomeningeal Metastases
Unlock the power of advanced diagnostics with CNSide™. Our cutting-edge assay provides unparalleled insights into tumor cells and circulating tumor DNA in cerebrospinal fluid, offering a new standard in patient care. *
Accurate
Comprehensive
Innovative
Patient-Centric
About CNSide™ Cerebrospinal Fluid Assay
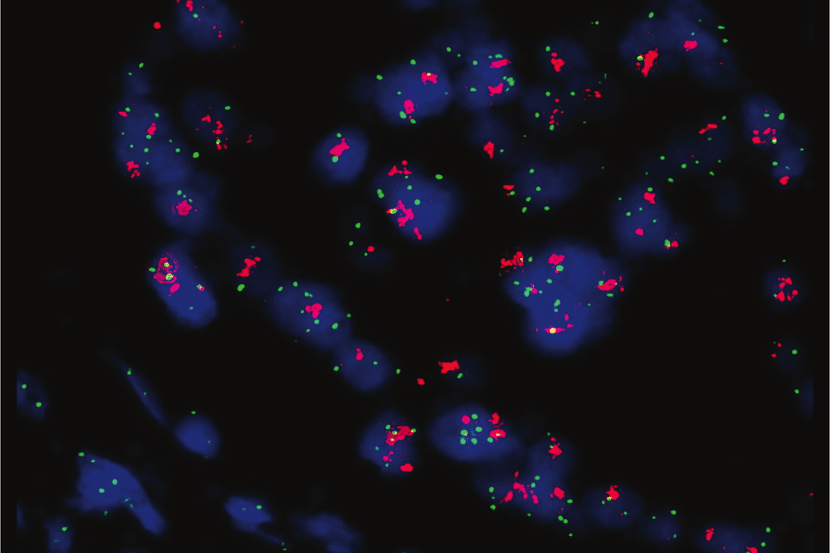
The CNSide™ Cerebrospinal Fluid (CSF) assay is a groundbreaking diagnostic tool designed to assess tumor cells that have spread from a primary carcinoma or melanoma to the central nervous system. By analyzing CSF to detect / quantify tumor cells and identify cellular biomarkers using ICC (immunocytochemistry) and FISH (fluorescence in situ hybridization), CNSide™ provides critical information that aids in the effective management of patients with Leptomeningeal Metastases (LM). Our goal is to enhance patient outcomes through precise and actionable insights. *
Step-by-Step Process
The CNSide™ CSF assay employs a meticulous methodology to ensure accurate and reliable results.
1. Sample Collection: CSF is collected via Standard of Care methods (lumbar puncture or Ommaya reservoir) from the patient.
2. Cell Isolation: Tumor cells are isolated and enriched from the CSF.
3. Molecular Analysis: Advanced techniques are used to analyze the molecular characteristics of the tumor cells and circulating tumor DNA. *
4. Data Interpretation: Detailed results are generated and sent to the ordering physician.

Key Features of CNSide™ CSF Assay
Precision Diagnostics
CNSide™ offers unparalleled precision in identifying tumor cells in the CSF, helping physicians form a Leptomeningeal Metastases diagnosis and aid in guiding treatment decisions.
Molecular Characterization
Our assay provides detailed molecular characterization of tumor cells, offering insights into the genetic makeup of the cancer for targeted therapy. *
Quantitative Analysis
CNSide™ enables quantitative analysis of tumor cells and circulating tumor DNA, aiding in the monitoring of disease progression and treatment response. *
Unmatched Precision
CNSide™ CSF Assay Benefits
Enhanced Detection
Accurately identifies tumor cells and circulating tumor DNA in CSF. *
Early Intervention
Facilitates timely clinical management decisions for better patient outcomes.
Comprehensive Analysis
Provides detailed molecular characterization of tumor cells. *
Quantitative Insights
Delivers precise quantitative data with the potential to guide treatment plans. *
Therapy Response Monitoring
Enables ongoing assessment of treatment efficacy and disease progression.
Outpatient Procedure
Collect CSF samples via lumbar puncture or Ommaya reservoir.
Personalized Treatment
Supports tailored therapeutic approaches based on individual molecular profiles. *
Clinical Confidence
Empowers healthcare providers with reliable data for informed decision-making.
CNSide™ CSF Assay: By the Numbers

%
Sensitivity in detecting tumor cells in patients with Leptomeningeal Metastases (Sweed et. al. 2023)

%
High specificity, with no tumor cells detected in patients without Leptomeningeal Metastases (Sweed et. al. 2023)
%
Therapeutic selection decisions influenced by actionable mutations identified by CNSide™ in CSF (Kumthekar et. al. FORESEE data)

%
Cases in which CNSide™ influenced treatment decisions (Kumthekar et. al. FORESEE data)
Frequently Asked Questions
Find answers to common questions about the CNSide™ CSF assay and its applications.
What is the CNSide™ assay?
The CNSide™ CSF assay is a diagnostic tool that identifies and analyzes tumor cells and circulating tumor DNA in cerebrospinal fluid, aiding in the management of leptomeningeal metastases.
How does the CNSide™ assay work?
The assay uses advanced molecular techniques to detect and quantify tumor cells and DNA in cerebrospinal fluid samples, providing detailed insights for clinical decision-making.
Who can benefit from the CNSide™ CSF assay?
Patients with leptomeningeal metastases from carcinomas or melanomas can benefit from the precise and comprehensive analysis provided by the CNSide™ CSF assay.
Does the CNSide™ CSF assay require a procedure?
Yes, the CNSide™ CSF assay requires an outpatient procedure to collect a patient’s cerebrospinal fluid via lumbar puncture or Ommaya reservoir.
How does the CNSide™ assay improve patient outcomes?
By providing accurate and detailed molecular data, the CNSide™ assay helps healthcare providers make informed treatment decisions, leading to better patient outcomes.
Where can healthcare providers get the CNSide™ CSF assay?
The CNSide™ CSF assay will be available through CNSide Diagnostics, LLC, a subsidiary of Plus Therapeutics, Inc., in early 2025. Contact us for more information on how to access this novel CSF assay platform.
How can patients with Leptomeningeal Metastases have their CSF evaluated by CNSide™?
Patients should contact their healthcare provider: neuro-oncologist, medical oncologist, or neuroimmunologist.
* CNSide Assay Estimated Availability: Tumor Cell Enumeration (TCE) Early 2025; ICC and FISH Mid 2025; NGS Late 2025
Discover the Power of CNSide™
Unlock critical insights into leptomeningeal metastases with the CNSide™ assay. Dive deeper into how our advanced diagnostic tool can revolutionize patient care. Contact CNSide Diagnostics today for more information.
